Premium Information is available for this item - Upgrade for $1 a day
6515-01-275-9139
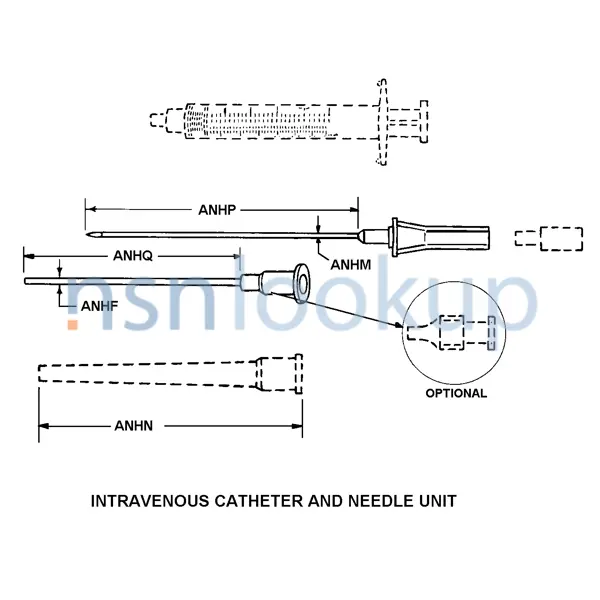
Intravenous Catheter And Needle Unit
6515012759139 012759139 SR87-1313-A
An item consisting of a cannulated needle with an intravenous catheter, and it may contain additional components, such as a hypodermic syringe, needle guard, flow control plug, and the like. The catheter is inserted into the vein by way of the needle's puncture, and is left in position for continued intravenous administration after the needle is withdrawn. A syringe, when supplied, is used to better control insertion of the catheter needle unit or for blood aspiration. View more Intravenous Catheter And Needle Unit
![]()
January 2023
6
 Marketplace 6515-01-275-9139
Marketplace 6515-01-275-9139
Request a Quotation from participating marketplace vendors
 Related Documents 6515-01-275-9139 2+ Documents (More...)
Related Documents 6515-01-275-9139 2+ Documents (More...)
Intravenous Catheter And Needle Unit 6515-01-275-9139 6515012759139 012759139 is an item consisting
Intravenous Catheter And Needle Unit SR87-1313-ARNCC: 3 | RNVC: 2 | DAC: 4 29 Mar 1988 6640-01-
 Restrictions 6515-01-275-9139
Restrictions 6515-01-275-9139
6515-01-275-9139 is a Intravenous Catheter And Needle Unit that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. The precious metals content of this item is unknown.
 Import and Export 6515-01-275-9139
Import and Export 6515-01-275-9139
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-01-275-9139
End Users 6515-01-275-9139
- MOE Rule:
- V540
- Effective Date:
- 1 Apr 1991
 Approved Sources 6515-01-275-9139
Approved Sources 6515-01-275-9139
- Part Number
- Manufacturer
- Status
- SR87-1313-A
- Manufacturer
- 60060 - Abbott Laboratories Inc. (Active)
- Primary Buy
- Primary Buy
 Datasheet 6515-01-275-9139
Datasheet 6515-01-275-9139
- Characteristic
- Specifications
- FIIG
- Specifications
- A22100
- Design Designation [ANEH]
- Intravenous
- Features Provided [CBBL]
- Sterile And Disposable
- Needle Nominal Diameter [ANHM]
- 17.000 Gage
- Nominal Diameter [ANHF]
- 18.000 Gage And 14.000 Gage
- Material And Location [ANNQ]
- Plastic Hub And Plastic, Polytetrafluoroethylene Catheter And Steel, Corrosion Resisting Needle
- Special Features [FEAT]
- Individual Package; 5 Inch Pressure Tubing; Female Winged Luer Lock Connector; Vented Cap; Cannulated Needle W/Needle Cover,Hub,And Guard; Ring Hub
- Tip Type [ANFG]
- Beveled
- Style Designator [STYL]
- Intravenous Catheter And Needle Unit
 NATO Stock Numbers Related to 6515-01-275-9139
NATO Stock Numbers Related to 6515-01-275-9139
 Freight Information 6515-01-275-9139
Freight Information 6515-01-275-9139
6515-01-275-9139 has freight characteristics.. 6515-01-275-9139 has a variance between NMFC and UFC when transported by rail and the description should be consulted.